Some of the greatest challenges in the medical technology industry for organisations are regulatory compliance, business partnership, and quality. Hear what we have to say about them
Challenge #1 – Regulatory Compliance – The Proverbial Itch Behind a Company’s Back
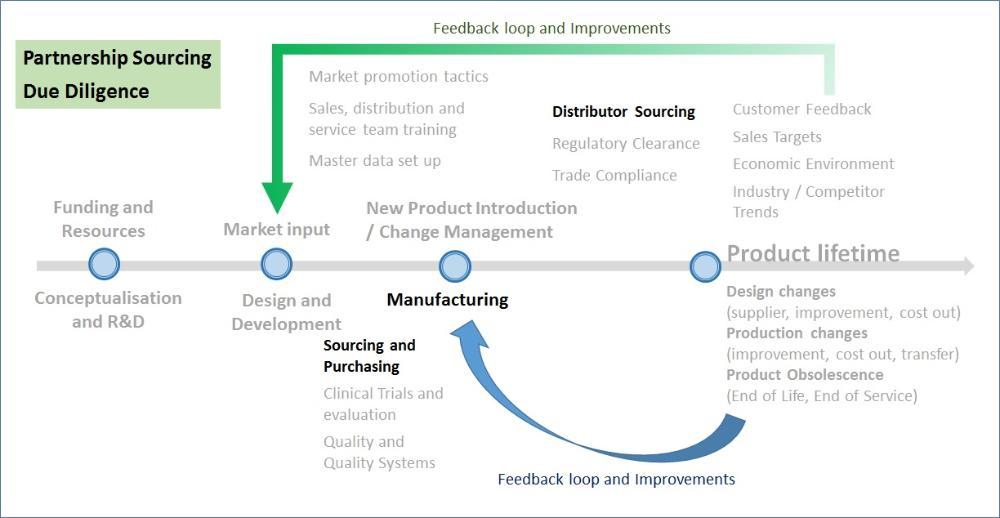
Companies are already facing an uphill task to innovate products and workflow in healthcare – whether if it is price competition in development, manufacturing and distributing, or challenges within the company to minimise overheads leads to targets to reduce cost centres to focus on revenue-generating resources.
Someway, somehow, companies find a way out of these issues. But what is the one issue that always flares up as a bad itch on your back that you can just never seem to reach? How many times does regulatory compliance come up in a business meeting, where a company is concerned about the time to market to launch a product, or that they cannot import due to some missing requirements by the authority? What is even more infuriating is that these issues keep coming up over and over again. While large, more established companies have the resources to hire regulatory or trade compliance professionals, smaller companies just don’t have that luxury to hire enough talent, or hire at all.
One of the Greatest Challenges in Market Entry is Regulatory Compliance. Regulatory and trade compliance professionals are currently in very high demand.
The fact that the resource demand is higher than supply leads to higher costs for companies to maintain a regulatory department. They need to handle unique pre-market product approvals in a quick and efficient manner, to gain market share and provide access to healthcare for the population. Resources need to have strong technical knowledge on product registration per country. They need good knowledge on the establishment and implementation of post market surveillance systems, quality systems in compliance with various regulations, FDA QSR as required, 13485, dealing with globalised Post Market Surveillance frameworks amongst the regulators and even US/CAN/EU/EMEA/LaTAm regulations.
Changing regulatory and trade compliance requirements forces companies to search for various avenues to be kept updated, or they will face nasty surprises. This translates to expenses to travel to networking meetings, training, etc. to maintain competency and expertise. Again, such needs put small to medium sized companies at a disadvantage, where either due to financial limitations or lack of education, such investment takes a backseat.
As companies look to outsource, they are faced with a gradually maturing consultancy market that is just only starting to provide the fundamental and immediate needs of healthcare companies. But what still lags – and troubles – many companies are on change management of the approved products, where changes can occur at any moment, and this change management cycle lasts as long as the product is in the market. The realisation that the product lifecycle needs to be managed and tied to regulatory/trade compliance, is the main reason why companies still need to maintain internal resources for market access. Is there a way to completely outsource?
There are several other challenges that are internally focused, internal processes of compiling, sending, and managing deliverables from manufacturer to distributor to regulator leading to approval, he alignment of company’s master data with regulatory, and trade compliance requirements. Often times these processes and its necessary infrastructure receives little investment and often overlooked as a key component in new product launches. Regulators are regularly frustrated with poor quality of submissions, leading to longer lead times to approval. To make things even more complicated, companies face very dynamic scenarios for each and every import into the markets, with frequent delays in clearing customs.
Why be Reactive? Be Proactive
We offer regulatory turnkey solutions to create the ‘peace of mind’ for you. Talk to us to learn how we manage regulatory compliance differently to ease your market entry barriers.
Challenge #2 – Business Partnership: The Importance of Business Due Diligence
A serious relationship involves significant investment by both parties. If two persons are in a serious relationship, they will go on dates to get acquainted and understand each other’s character, behaviours better. They’ll seek to understand each other’s outlook to the various aspects of life. They spend time together, understand each others’ backgrounds well, and perhaps go through some challenging times together. All that has to happen, before considering to tie the knot. Fair and reasonable, right?
A Business Partnership is a Serious Relationship

Isn’t it so? There’s so much money involved, as well as time, effort, resources invested.
One failed start means finding another company and having to do everything all over again. Think of the loss of the time to market. Isn’t it better to be sure than to be sorry down the road?
Isn’t it common to hear stories (and some horrific ones) about how businesses failed because they partnered with the wrong guys?
Why this critical step of due diligence (which is somewhat like a pretty serious date) prior to signing up any business agreement not always in the forefront of most companies’ minds?
What is Due Diligence?
It is an investigation on a prospective business before signing a contract with them. This investigation is performed by making business-critical information available to the decision-makers who will systematically, carefully analyse all its costs, benefits, and risks, leading to an informed decision.
Get Everyone Involved to Make an Informed Decision

All these functional aspects need to be thoroughly evaluated whenever you’re considering a partner for manufacturing or distribution. Due diligence scheduling and timely involvement of other stakeholders in the due diligence process to enhance the competency pool is very important in the final decision-making process.
Here’s where the business due diligence process can positively impact your commercialisation efforts – right in the beginning

Having to find and partner with another business for commercialisation is extremely expensive and resource intensive. It just can’t be an expensive mistake.
We perform the due diligence – and you can learn along the way
Access-2-Healthcare can arm you with the knowledge and competency to execute a due diligence exercise effectively. There may be templates and theories out there that you can source, but we put all of what you need (and only what you need), in a practical, usable manner.
We can physically, objectively and systematically assess and qualify manufacturers and distributors through background check, and off-site/on-site business due diligence.
We can assess the business risks together with you for any business partnership
Email us to find out how due diligence can benefit your organisation.
Challenge #3 – Greatest Challenges in Healthcare Quality Today
Quality touches almost every aspect of the Product Lifecycle
However, Quality and Quality Systems have become almost a ‘necessary evil’ in any business,
“it can be done later”
“We have to tick the box”
and especially in Healthcare… “we need to satisfy the ‘healthcare policemen”- namely regulators
New entrants to healthcare struggle to make sense of their intents – what it is, why is it needed – going beyond just satisfying auditors and regulators.
Large healthcare organisations struggle with holding on to their intents, due to increasing complexity from the increasing complex business models, mergers and acquisitions, different levels of competency, introducing new blood from outside the healthcare industry to drive innovation. These regulators know if Quality and a Quality System are in place, then it’s likely any product would be inherently safe. A whole host of standards and regulatory requirements gradually developed, and created an entire industry segment just focused on meeting these standards and requirements!
Here’s how we help you through Quality Consulting