Nifty Tips on calculating Product License renewal timelines for the CFDA
On September 26, 2016, the CFDA Administrative Services issued a statement on applicable information with regards to the renewal of the product registration license for medical devices (No. 179), clarified that the submission time of application for the renewal registration of medical devices is the actual processing date.
What does this mean?
Using the reference of the date 6 months prior to license expiry is not correct. Having high quality submissions for this license renewal application is absolutely critical.
Referring to the original regulations within the Guidance for for the Registration of Medical Devices (including vitro diagnostics) – if the expiration of the expiry date of the Medical Device Registration Certificate needs to be renewed, the registrant should apply to the food and drug supervision and administration department for registration 6 months before the expiry date of the Medical Device Registration Certificate and submit the application materials according to the relevant requirements. The submission time of application materials mentioned above refers to actual date of application for renewal of registration.
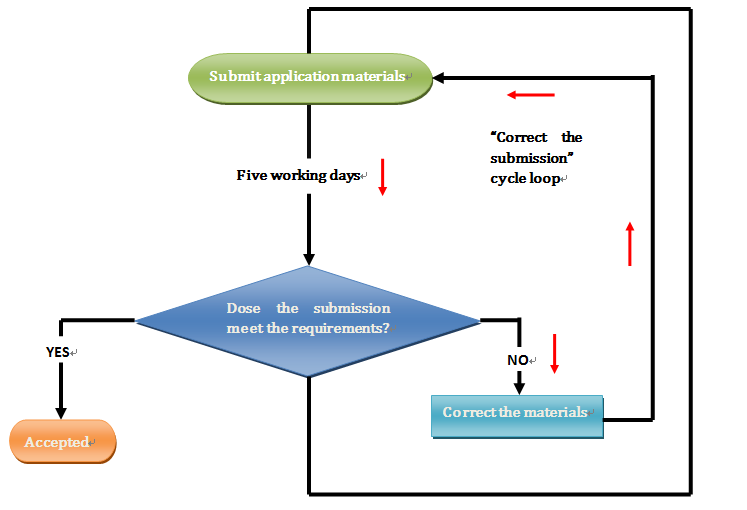
However, it takes five working days before CFDA reviews the application and acknowledges that the submission has been accepted. Thus the the application for this renewal becomes 6 months +5 working days (review).
If the application does not meet the requirements, the applicant will need to correct the application materials and submit again.
Let’s assume this time to be 3 days. Then the time extends to 6 months +5 working days (review) +3 days (materials preparation) +5 working days (re-review). If the number of correcting materials more than once, then add 5+X+5 days per cycle
Therefore, if you wish to ensure that your product license is renewal on time the quality of the information within the submission is critical. If insufficient time is budgeted and the quality of submission is poor, you may very well end up in endless review cycles and miss the boat for product renewal!
This provision shall be implemented from January 1, 2017. So for those of you whose registration certificate is valid until in the middle of 2017, it is recommended to start in advance to apply for renewal of registration items!