China FDA – Don’t Miss the Boat!
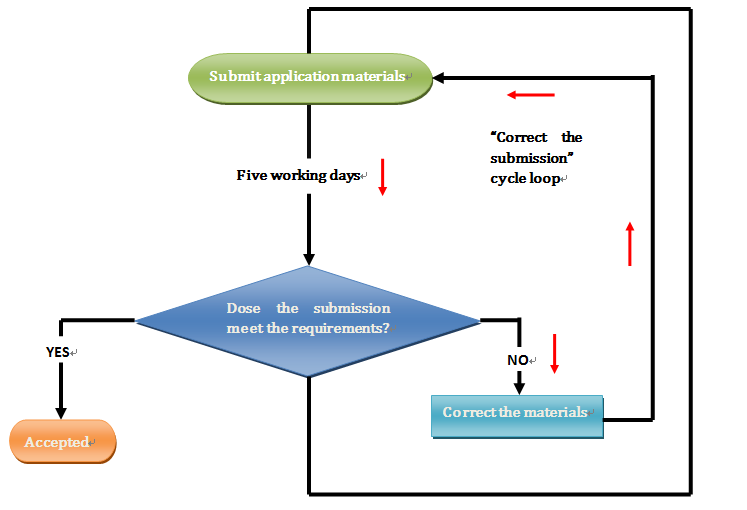
Nifty Tips on calculating Product License renewal timelines for the CFDA On September 26, 2016, the CFDA Administrative Services issued a statement on applicable information with regards to the renewal of the product registration license for medical devices (No. 179), clarified that the submission time of application for the renewal registration of medical devices […]