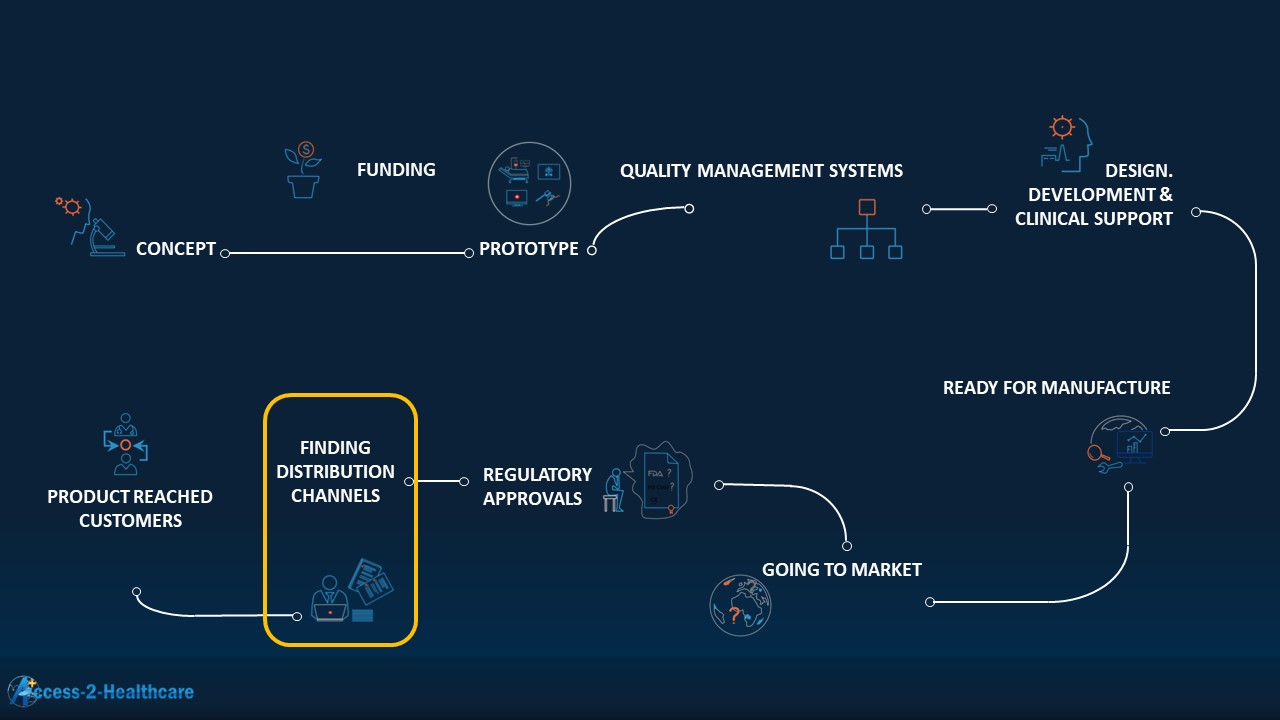
Sales distribution channels are always needed, it’s just that they are not necessarily distributor channels.
What we always want a distributor to have are:
- Clinical network, which leads to sales.
- Human resources - for business development, sales pitching, and follow up
- Market entry, logistics – a party that will absorb the costs of these activities
However, the most important factor that a distributor needs to have is : commitment.
Without commitment,
- Product knowledge cannot be acquired due to lack of time, human resources allocated.
- Business development efforts may not be as comprehensive
- Follow up may be slow, to a point where the lead turns cold
- Market entry efforts may be slow
It most cases, Commitment isn’t easy to come by when the distributor
- Finds your product ‘interesting’ and ‘let’s give it a go’
- Does not provide a clear go-to-market strategy, and intends to just ‘integrate with our current offerings’
- Has similar products to yours
Can we do without a distributor?
You can do without a distributor, but you cannot do without sales distribution. To build these channels, commitment from the manufacturer is the most important factor – to be present in the market before the product gains market entry, building awareness, and gaining more knowledge about the market dynamics in the country.
There are situations where a distributor would really be needed. Not just their existing network, but their technical service support, or storage space. But even that, there may be ways around it. Email us to find out how.
Still clear as mud?