Company X tries to launch product Y in Country Z. Regulatory Affairs compiles documents for Country Z, only to realise some documents are not available. Regulatory Affairs checks with the Design team, only to find out the Design Team didn’t even perform certain tasks, never mind no documents!
Sounds familiar? How did this happen?
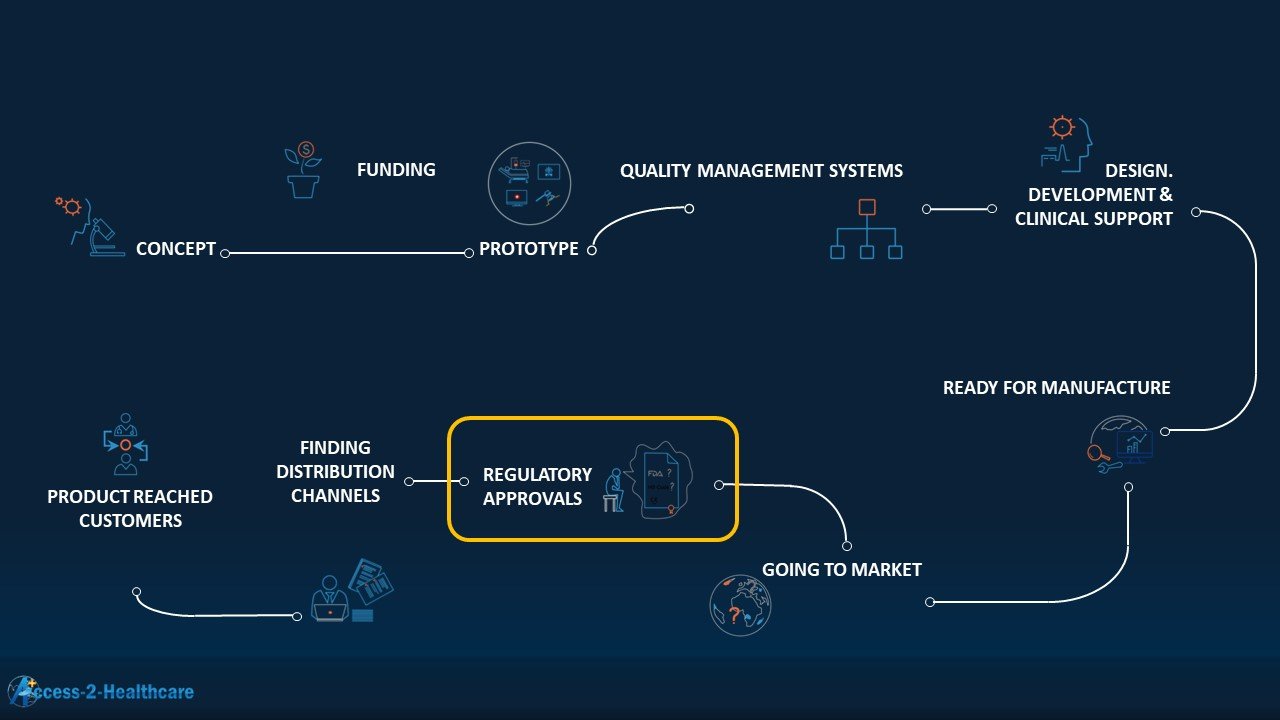
All regulatory dossiers are a subset of design and development documentation. Design and Development Documentation can only exist if the design and development activities, including clinical and usability activities, are performed!
To make things worse, ISO, QSMR, even MDSAP auditors didn’t have time, or mental bandwidth to audit design files in detail (legally acceptable as all audits are a sampling inspection). And no one really listens to the quality manager.
In some other (improved) cases, you may have marketing and management working with the regulatory affairs functions in go-to-market strategy sessions. Regulatory affairs proceed to provide regulatory requirements of the countries of interest. Sounds logical, but requirements are not specific document checklists, and such sessions happens way after the design effort has progressed ahead, thus having all forms of unwillingness to repeat anything or perform additional tasks.
What is really needed, in this order:
- A best guess of which countries to launch
- Collate regulatory requirements, determine specific documentation (technical and otherwise) to gather
- Identify clear engineering and clinical requirements (power socket, power supply, local clinical studies needed etc)
- 4. Create comprehensive document checklist based on the preliminary countries to launch in #1
- 5. Share examples of what these documents are. Work with design/development to TRANSLATE what this means for them. This is because design/development teams cannot be forced to generate design documents according to what regulatory affairs wants, with clear exceptions of specific documents such as the General Safety and Performance Requirements (GSPR) or Essential Requirements, or Clinical Evaluation Reports.
This approach will allow potential issues to surface earlier and find areas of resolution. Such an approach will also foster mutual learning of each departments’ discipline and develop necessary empathy to facilitate market launch successfully.
Still clear as mud?